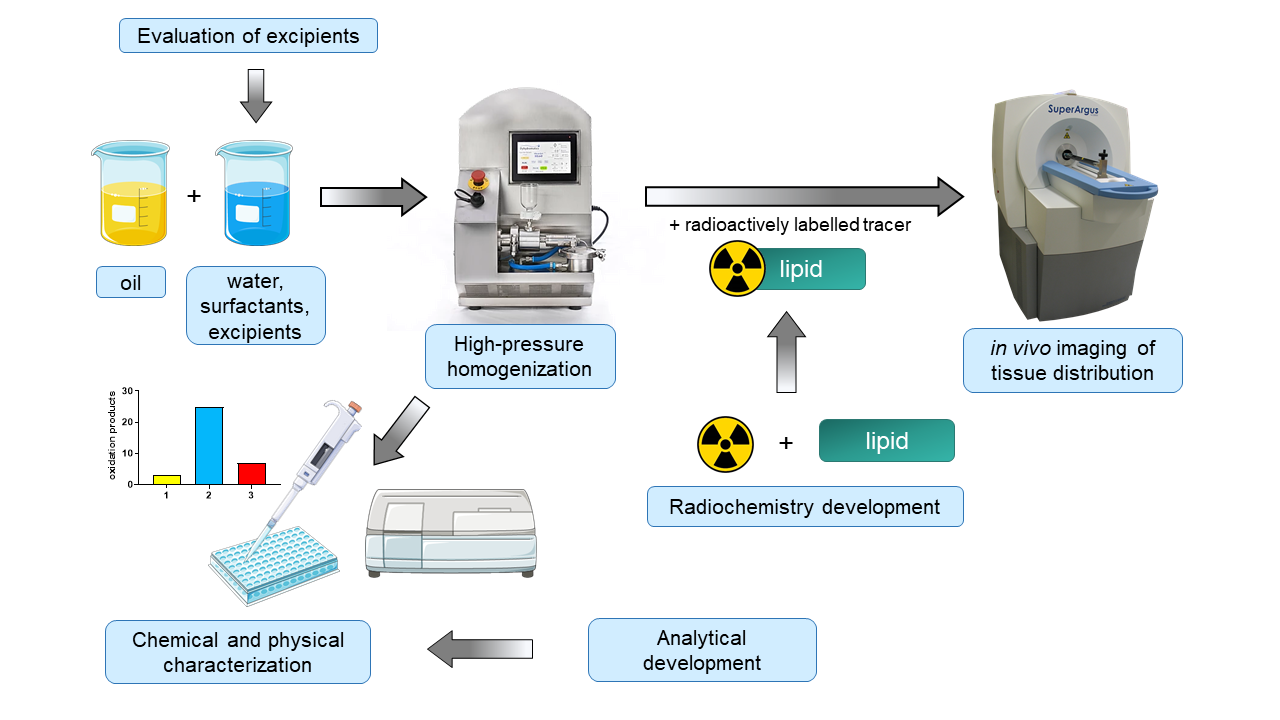
Formulation optimization of lipid emulsions for improved parenteral nutrition therapy
Currently available lipid emulsions for parenteral nutrition are associated with considerable inflammatory adverse effects when used over longer time periods. We thus aim at optimizing the lipid composition as well as a more balanced tissue distribution of lipid emulsions. Emulsions are manufactured by high-pressure homogenization, sterilized by autoclaving and then characterized with in-house developed assays for lipid oxidation products, optimized for minimal sample consumption and high sample throughput. So far, we identified a lead composition meeting the quality requirements in terms of formation of oxidation products. Moreover, it elicits a favourable inflammatory marker profile in vivo. Through the addition of trace amounts of radioactively labelled lipids to the emulsions, we can follow the tissue distribution. We observed a distinct distribution behavior compared to an established reference product.
We can conclude that the lipid composition is at least in parts responsible for the formation of inflammatory mediators. By exchanging the oil source, this process can be prevented.
Efforts will now continue to develop a radioactively labelled lipid to image the time-resolved tissue distribution in more detail.
This project is funded by a external page Sinergia grant by the Swiss National Science Foundation.
Literature: Lucchinetti, E. et al. Novel lipid emulsion for total parenteral nutrition based on 18-carbon n–3 fatty acids elicits a superior immunometabolic phenotype in a murine model compared with standard lipid emulsions. ACJN. 116 (6), 1805–1819 external page (2022).